Scandinavian Biopharma shows strong financial results for the pandemic year 2020
The year we have left behind us has been different in many ways. Despite the major impact the pandemic had on operations in 2020, Scandinavian Biopharma reports largely unchanged revenue for the distribution operations and important progress has been made in the development of the ETEC vaccine candidate ETVAX®.
Our world-leading position in diarrhoea vaccine against ETEC has been further strengthened and the clinical development program has continued to show good progress in 2020. A total of seven studies have now been performed on humans in the USA, Bangladesh, Sweden, Finland and Zambia with very promising results.
In 2020, two promising study results were presented; the Phase 2b study on travellers in Benin and the Phase 1 study on children from 6 months in Zambia. The Benin study confirms ETVAX® excellent safety and strong immune response and a significant protective effect of 56% against all severe diarrhoea, regardless of pathogen, which is significantly better than we dared to hope for. In the safety and immunogenicity study in Zambia, the vaccinations of all participants were successfully completed and the samples are currently being analysed. The results are expected to be completed during the first half of 2021.
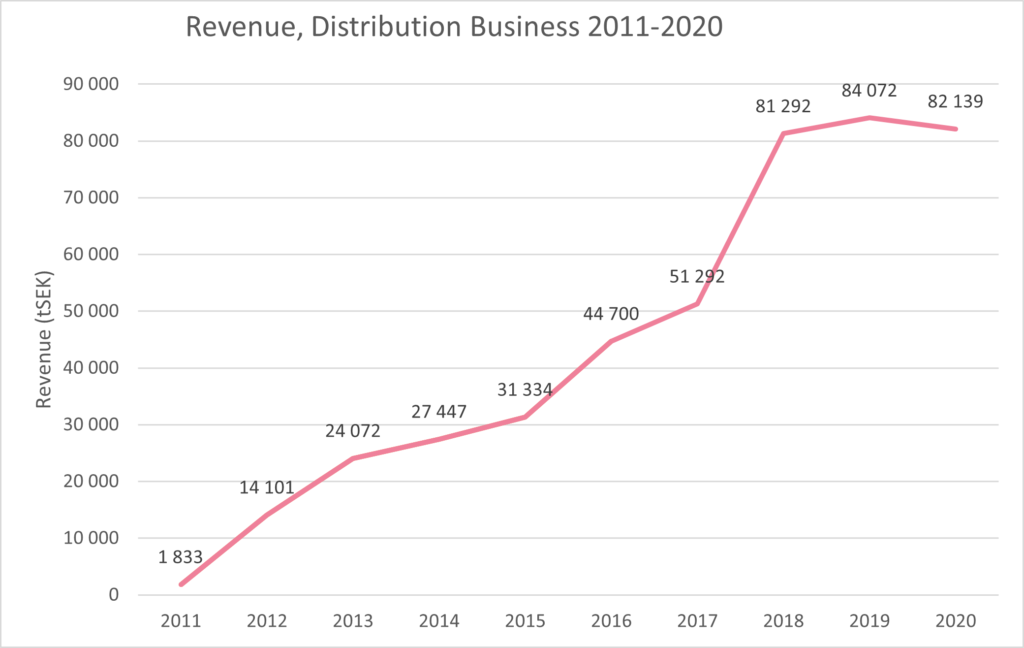
“We are very happy that we have strengthened our organization in 2020 and welcomed so many new faces, both in manufacturing, QC, regulatory, clinical and sales. Distribution operations have continued to develop well despite the impact of the pandemic. In 2020, we have both renewed and signed a number of new distribution agreements. Sales for Scandinavian Biopharma Distribution decreased slightly from 84.1 MSEK (7.9 MEUR) in 2019 to 82.1 MSEK (7.8 MEUR) in 2020, which corresponds to 2%. But we have a continued underlying growth in our distribution portfolio and the decrease we see is directly linked to our travel-related products that have been negatively affected by the pandemic”, says Peter Liss CFO at Scandinavian Biopharma.
In 2020, important financial grants/agreements were also signed to enable the development of a tailor-made vaccine formulation of ETVAX® for children in low- and middle-income countries. The grants also make it possible to carry out our planned phase III studies in Africa and finance manufacturing and development activities for ETVAX®.
“It is absolutely fantastic that we received an additional grant of a total of 10.6 MEURO from the EU via EDCTP and after our positive study results from Benin, we entered into an agreement with USAMRAA where the goal is to accelerate the approval of our vaccine in the US,” says Björn Sjöstrand CEO of Scandinavian Biopharma.
Another milestone in the previous year was that the World Health Organization’s expert committee, PDVAC (Product Development for Vaccines Advisory Committee), determined that ETEC would remain a priority pathogen as the bacterium is one of the leading causes of diarrheal disease, especially among children in low- and middle-income countries, but also for travellers.
“Due to the pandemic, we will also face continued challenges in 2021. A big thank you to all our employees and partners, for quickly adjusting, adapting and finding new and safe ways of working to master the situation, says Björn Sjöstrand who in 2020 was named one of the Stockholm finalists, in one of the world’s top awards for Entrepreneurs, EY Entrepreneur Of The Year.