Scandinavian Biopharma reports 27% growth for 2023 and initiates a pre-study ahead of phase III
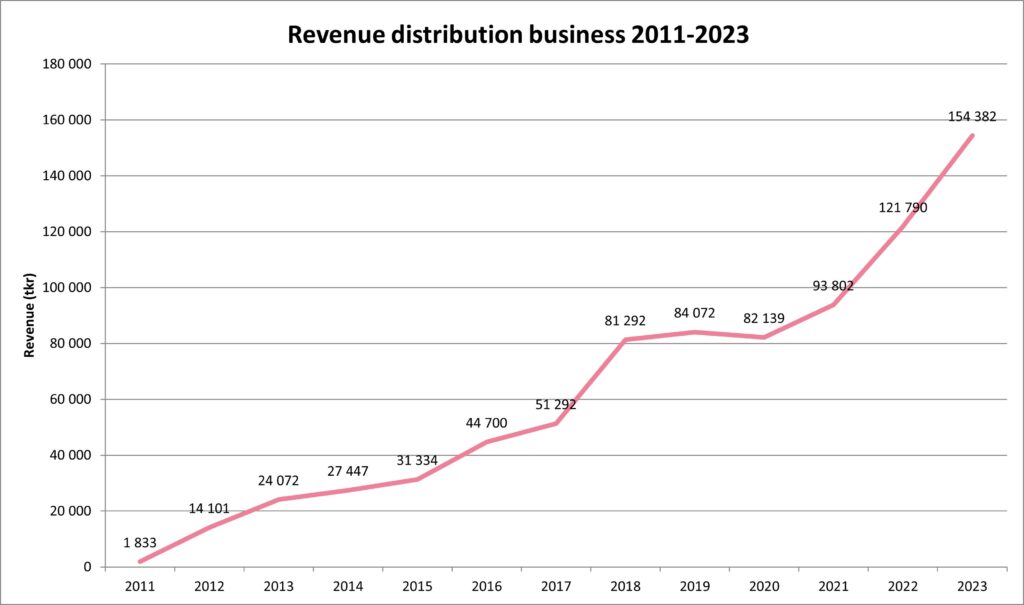
Scandinavian Biopharma is pleased to announce another year of significant growth. For the full year 2023, the revenue increased by over 33 MSEK, corresponding an organic growth of 27%. The total revenue for 2023 amounted to 154 MSEK.
The ETEC vaccine candidate is well on its way into phase III. During 2023, there was a strong focus on preparing a pre-study for a planned phase III CHIM (Controlled Human Infection Model) study in the USA. The pre-study, which started at the end of February 2024, aims to verify the attack rate of the proposed challenge dose.
The previous year also saw the completion of the company’s phase IIb study in Gambia, involving 4.936 children aged 6-18 months. The study evaluated the protective effect against moderate to severe diarrhoea caused by ETEC. Preliminary results from the study look promising and are expected to be presented in the second quarter of 2024.
Significant attention during 2023 was also given to vaccine manufacturing and interactions, particularly with the FDA. Evidence that Scandinavian Biopharma has a very promising vaccine candidate and a well-developed plan for its continued development program is that the company was awarded an additional 5 million USD from the U.S. Army Medical Materiel Development Activity (USAMMDA) and EUR 1.7 million from EDCTP in 2023.
The company’s distribution business, which includes a wide range of specialty biopharma products with a focus on vaccines and immunoglobulins, continued its growth journey in 2023. Growth was primarily driven by new products. During the year, the product portfolio was expanded to include Food for Special Medical Purposes (FSMP) for children with cow’s milk allergy. The commercial team expanded to include local presence also in Finland, Norway, and Denmark. With the increased presence in the Nordic countries, the company is now even better equipped to continue growing and strengthen its position as a leading supplier of vaccines and specialty biopharma products.
Scandinavian Biopharma is now preparing for upcoming phase III studies in both travellers and children in low- and middle-income countries for its ETEC vaccine candidate. The goal is to register the world’s first vaccine against diarrhea caused by ETEC. A vaccine for both children and adults in endemic countries as well as for travellers to risk areas for travellers’ diarrhoea. 2024 will be an exciting year!