Scandinavian Biopharma presents the year-end report for 2022 with a 30% growth, record sales and the ETEC vaccine candidate about to enter phase 3
It is with great pleasure that we present another really good year of growth. The figures for the full year 2022 add up to an increase in sales of just over SEK 28 million, equivalent to 30% in organic growth. In total, sales amount to approximately SEK 122 million. Our ETEC vaccine candidate, ETVAX, is about to enter phase 3 and a major focus in 2022 has been to build up production capacity, in cooperation with contract manufacturers, to prepare for commercial production of ETVAX. This has meant a continued and significant strengthening of the company’s capabilities and organisation, especially in manufacturing related functions.
During the autumn we successfully completed the vaccination of 5,000 children in a Phase 2b study in The Gambia. This study evaluates the protective efficacy against moderate to severe diarrhoea caused by ETEC in children in an endemic country. A follow-up period for passive surveillance is ongoing until October 2023. Another milestone was the immunogenicity study that we carried out in cooperation with the University of Gothenburg during 2022. In this study we showed that our user-friendly commercial formulation is equivalent to the formulation we tested in previous studies”, says Nils Carlin, responsible for the vaccine design.
A confirmation that we have a very promising vaccine candidate and a well-developed plan for our remaining development programme is the extension of our agreement with the U.S. Army Medical Materiel Development Activity (USAMMDA) in 2022. This means that we have received additional funding for the development of our world-leading ETEC vaccine candidate with the aim of accelerating the development and authorisation of the vaccine in the US.
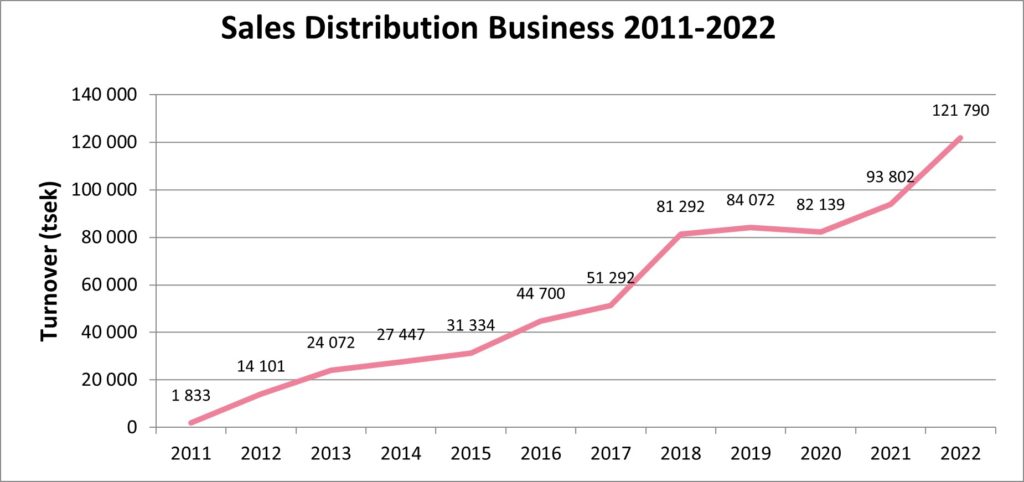
Our distribution business, which consists of a wide range of specialty biopharma products with a focus on vaccines and immunoglobulins, continued its growth path in 2022 showing solid profitability. We set a new sales record and increased from SEK 93.8 million in 2021 to SEK 121.8 million in 2022. Growth was driven by both geographical expansion and new product launches.
During 2022, we have further strengthened our position as a leading diarrhoea vaccine company by expanding our pipeline with a Campylobacter vaccine technology in-licensed from the Canadian company VaxAlta Inc. This is a collaboration that fits very well with our strategy in Global Health, the essential and life-saving role new vaccines play in the field of diarrhoeal diseases and how vaccines can reduce both human suffering and the imminent global threat of antibiotic resistance.
“In 2022, we have achieved a number of important milestones and now have a very exciting year ahead of us. We are preparing to start our phase 3 study in travellers while completing our large phase 2b paediatric study in The Gambia. We are getting closer and closer to our goal of registering the world’s first vaccine against diarrhoea caused by the ETEC bacteria, a vaccine that will be developed both for children and adults in endemic countries as well as for travellers to areas at risk of travellers’ diarrhoea”, concludes Björn Sjöstrand.

